Vision
Our vision is to harness the therapeutic potential of microRNAs, specifically miR-451a and let-7i-5p, to revolutionize the diagnosis, treatment, and prevention of hemolytic diseases such as SCD and malaria. By unraveling the molecular mechanisms underlying inflammation in these conditions, we strive to pave the way for personalized medicine approaches that improve patient outcomes and quality of life.
Objectives
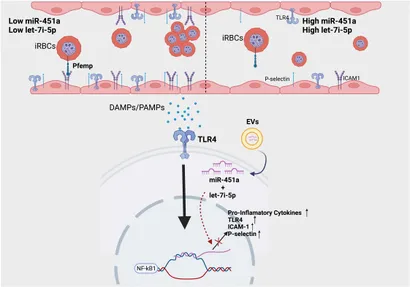
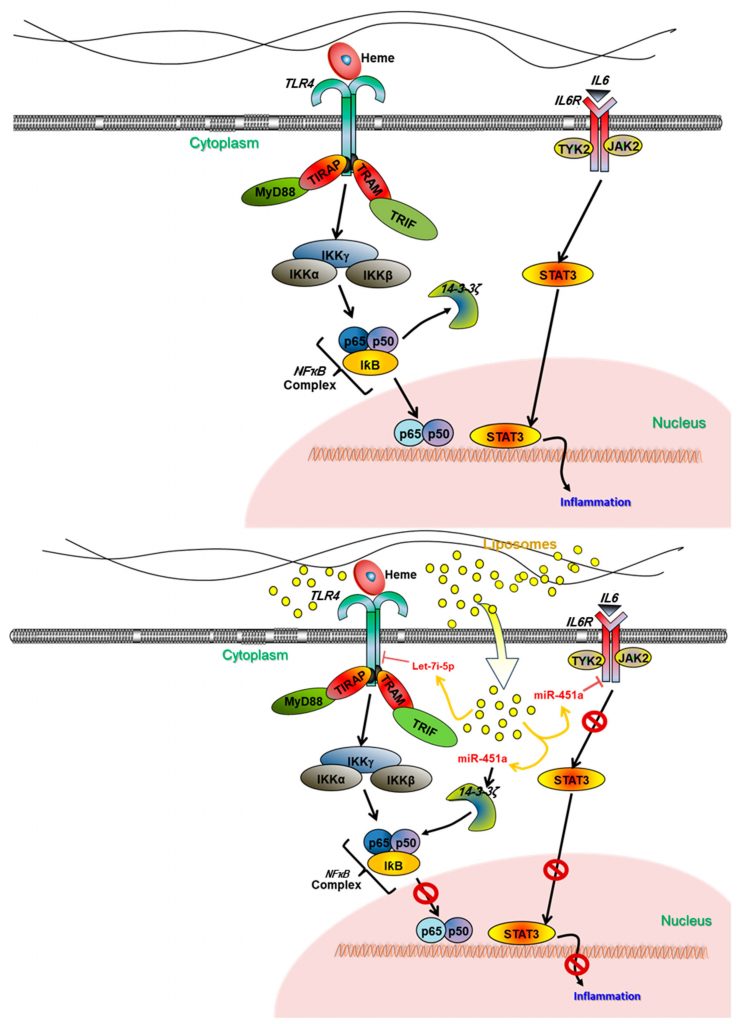
- 1. Investigate the Therapeutic Potential: We aim to elucidate the role of miR-451a and let-7i-5p in managing chronic inflammation in SCD. By exploring their regulation of heme-induced inflammation through the AKT/PI3K and JAK/STAT3 pathways, we seek to identify these microRNAs as promising therapeutic targets for mitigating inflammation-associated complications in SCD.
- 2. Biomarker Discovery: Our objective is to identify and validate exosomal miRNA biomarkers, particularly miR-451a and let-7i-5p, for SCD diagnosis and prognosis. Through functional studies on patient-derived cells and validation in a case-control patient cohort, we aim to establish these biomarkers as reliable indicators of disease progression and treatment response.
- 3. Impact Assessment: We endeavor to assess the effects of miR-451a and let-7i-5p on immune response using a humanized-hybrid sickle cell mouse model. By evaluating the therapeutic efficacy of these microRNAs in vivo, we aim to translate our findings into clinical applications that address the underlying mechanisms of inflammation in hemolytic diseases.
Collaborative Approach
Collaborating with esteemed experts such as Dr. Stiles at MSM, and Dr. Botchwey at GA-tech, Dr. Hood at University of Louisville, Prof. Wilson at the Noguchi Memorial Institute for Medical Research in the University of Ghana, we leverage interdisciplinary expertise to propel our research forward. By fostering collaborations with leading scientists and clinicians, we aim to synergize efforts and accelerate the translation of our discoveries from bench to bedside.
Contribution to Science
Our laboratory has made significant contributions to the understanding of SCD and malaria, elucidating the complex interplay between genetic factors, inflammation, and disease susceptibility. Through our comprehensive studies, we have identified inflammatory cytokines as potential biomarkers and explored novel therapeutic strategies, such as dietary interventions and microRNA-based therapies, for managing inflammation-associated complications in hemolytic diseases.
Impact
By advancing knowledge and developing innovative solutions, our research has the potential to transform clinical practice and improve the lives of individuals affected by hemoglobinopathies worldwide. Through our collaborative efforts and translational approach, we strive to make meaningful contributions to the diagnosis, treatment, and prevention of SCD and malaria, ultimately enhancing healthcare outcomes and reducing the global burden of these devastating diseases.
Global Health Engagement
At the forefront of our research endeavors lies a deep commitment to global health equity and collaboration. Recognizing the urgent need to address health disparities and challenges faced by vulnerable populations worldwide, our laboratory actively engages in initiatives aimed at advancing knowledge, building capacity, and improving health outcomes in low-resource settings.
Research Focus
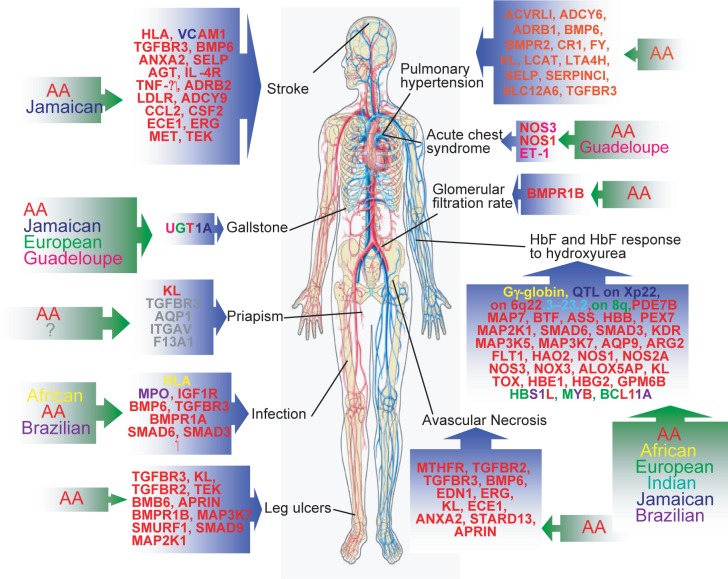
Our research focuses on the intersection of hemoglobinopathies, particularly sickle cell disease (SCD), and malaria, two diseases that disproportionately affect populations in sub-Saharan Africa and other regions with limited access to healthcare resources. By investigating the molecular mechanisms underlying these diseases and exploring innovative interventions, we seek to alleviate suffering and improve the quality of life for individuals and communities affected by these conditions.
Capacity Building
Central to our global health mission is the belief in the power of capacity building and knowledge exchange. Through collaborative partnerships with institutions and researchers in resource-limited settings, we aim to empower local communities by providing training, mentorship, and support in research methodology, data analysis, and scientific communication. By equipping local scientists and healthcare professionals with the tools and skills needed to address pressing health challenges, we strive to foster sustainable solutions and promote self-reliance.
Community Engagement
We recognize the importance of community engagement in driving meaningful change and ensuring the relevance and impact of our research efforts. Through participatory research approaches, we actively involve community members in the design, implementation, and evaluation of our projects, thereby ensuring that interventions are culturally appropriate, acceptable, and effective. By partnering with local stakeholders, advocacy groups, and policymakers, we aim to amplify the voices of those most affected by health disparities and advocate for policies and programs that promote health equity and social justice.
Policy Advocacy
Our laboratory is committed to advocating for evidence-based policies and interventions that address the underlying determinants of health and promote equitable access to healthcare services. By leveraging our research findings and expertise, we engage with policymakers, government agencies, and international organizations to influence policy decisions, mobilize resources, and prioritize investments in global health research and development. Through advocacy efforts, we strive to create an enabling environment for scientific innovation, collaboration, and impact.
Impact Assessment
We believe in the importance of rigorously assessing the impact of our research and interventions to ensure accountability, transparency, and continuous learning. Through monitoring and evaluation activities, we measure the effectiveness, scalability, and sustainability of our programs and interventions, identify areas for improvement, and share lessons learned with the broader global health community. By promoting a culture of evidence-based decision-making and learning, we aim to maximize our impact and contribute to positive health outcomes for all.
Join Us in Our Mission
We invite researchers, healthcare professionals, policymakers, advocates, and community members to join us in our mission to advance global health equity and improve health outcomes for all. Together, we can harness the power of science, collaboration, and innovation to create a healthier, more equitable world for future generations.
Research
Welcome to the forefront of biomedical research at our laboratory, where we delve into the intricate relationship between malaria, sickle cell disease (SCD), and hemoglobinopathies. Through cutting-edge studies, we aim to understand how genetic polymorphisms influence the severity of malaria in Ghanaian populations. Our project objectives revolve around unraveling the role of key genetic variants, such as HO-1, CXCL10/CXCR3, and STAT1/STAT3, in mediating host protection against severe Plasmodium infection. We employ advanced genomic and proteomic methods to identify biomarkers associated with malaria severity and investigate human copy number polymorphisms associated with severe malaria anemia. Additionally, we explore the therapeutic potential of microRNAs, specifically miR-451a and let-7i-5p, in modulating heme-induced inflammation, a common pathway in hemolytic disorders like malaria and SCD. By leveraging innovative techniques such as liposome-mediated delivery of miRNAs, we aim to mitigate heme-induced inflammation and pave the way for novel therapeutic interventions. Through our collaborative efforts and dedication to global health equity, we strive to make meaningful contributions to the understanding and management of hemoglobinopathies and malaria, ultimately improving health outcomes for vulnerable populations worldwide.
- 1. miRNA Therapeutic Research: Our laboratory is dedicated to investigating the therapeutic potential of microRNAs (miRNAs), specifically miR-451a and let-7i-5p, in managing heme-induced inflammation in hemolytic disorders like malaria and sickle cell disease (SCD). Through innovative approaches such as liposome-mediated delivery, we aim to develop novel interventions to mitigate inflammation and improve patient outcomes.
- 2. Biomarker Discoveries: We are actively involved in identifying and validating biomarkers associated with disease severity in malaria and hemoglobinopathies. By employing advanced genomic and proteomic methods, we seek to uncover molecular signatures that can predict disease progression and treatment response, ultimately facilitating personalized medicine approaches.
- 3. Exosomes and Microvesicles as Delivery Tools: Our research explores the use of extracellular vesicles, such as exosomes and microvesicles, as delivery vehicles for therapeutic molecules, including miRNAs. By harnessing the natural cargo-carrying capabilities of these vesicles, we aim to enhance the targeted delivery of therapeutic agents and optimize treatment efficacy while minimizing off-target effects.
- 4. Induced Pluripotent Stem Cells (iPSCs) and Organoids: We utilize induced pluripotent stem cells (iPSCs) as a cutting-edge platform for disease modeling, drug discovery, and regenerative therapeutics. By generating patient-specific iPSCs, we can recapitulate disease phenotypes in vitro and study the underlying mechanisms of hemolytic disorders, paving the way for the development of novel therapies.
- 5. Global Health Initiatives: Our laboratory is committed to addressing global health disparities by conducting research that has direct relevance to low-resource settings, particularly in malaria-endemic regions. Through collaborative partnerships with international institutions and local communities, we aim to develop sustainable solutions to improve health outcomes and build research capacity in these regions.
- 6. Biorepository of Samples Collected: We maintain a comprehensive biorepository of clinical samples collected from diverse populations, including individuals with malaria, SCD, and other hemolytic disorders. These samples serve as valuable resources for conducting translational research, biomarker discovery, and validation studies, facilitating collaborative efforts and advancing scientific knowledge in the field.
People

Adel Driss, PhD, Principal Investigator. Dr. Adel Driss, PhD, is a distinguished scientist and educator with a rich academic background in biology and genetics from the Faculty of Sciences of Tunis, Tunisia. Currently serving as an Assistant Professor in the Department of Physiology at Morehouse School of Medicine (MSM) in Atlanta, GA, Dr. Driss brings a wealth of experience to our laboratory. His expertise spans various facets of biomedical research, including molecular mechanisms underlying malaria protection in sickle cell trait individuals and heme-induced inflammation. Dr. Driss has demonstrated exceptional leadership in project management, strategic collaborations, and mentorship, directing numerous PhD and master’s students and fostering international partnerships with institutions such as the University of Ghana and the Pasteur Institute of Tunis. As a Fogarty Global Health Fellow, he conducted groundbreaking research in Ghana, shedding light on molecular factors contributing to malaria severity disparities. Dr. Driss’s commitment to advancing public and global health is evident in his extensive work on disease pathogenesis, therapeutic interventions, and community health promotion initiatives. With a strong foundation in research methodology, genetics, and parasitology, Dr. Driss is dedicated to addressing critical health challenges and improving outcomes for underserved populations worldwide.

Alaijah Bashi, PhD candidate. Alaijah Bashi, is a PhD and Master of Science in Clinical Research (MSCR) candidate at Morehouse School of Medicine. She showcases exemplary leadership and research skills in her pursuit of biomedical science. With a bachelor’s degree in Neuroscience from Michigan State University, she brings a diverse skill set to her current research on heme-induced inflammation in hemolytic disorders like malaria and sickle cell disease. Alaijah’s expertise in project management, research, and public speaking, complemented by her strong academic background, positions her as a valuable asset to her research team. Under the mentorship of Dr. Driss, she explores the potential of microRNA-loaded liposomes to mitigate inflammatory responses, offering promising therapeutic insights. Alaijah’s dedication to addressing global health challenges underscores her commitment to making meaningful contributions to biomedical research. Alaijah is a RISE and a CTSA-TL1 fellow. Bashi published 4 peer reviewed articles already, one of them being as a first author.
MiR-451a and let-7i-5p loaded extracellular vesicles attenuate heme-induced inflammation in hiPSC-derived endothelial cells. Thomas JJ, Harp KO, Bashi A, Hood JL, Botchway F, Wilson MD, Thompson WE, Stiles JK, Driss A. Front Immunol. 2022 Dec 22;13:1082414. doi: 10.3389/fimmu.2022.1082414. eCollection 2022. PMID: 36618355
MicroRNAs miR-451a and Let-7i-5p Profiles in Circulating Exosomes Vary among Individuals with Different Sickle Hemoglobin Genotypes and Malaria. Oxendine Harp K, Bashi A, Botchway F, Dei-Adomakoh Y, Iqbal SA, Wilson MD, Adjei AA, Stiles JK, Driss A. J Clin Med. 2022 Jan 19;11(3):500. doi: 10.3390/jcm11030500. PMID: 35159951
Sickle Cell Hemoglobin Genotypes Affect Malaria Parasite Growth and Correlate with Exosomal miR-451a and let-7i-5p Levels. Oxendine Harp K, Bashi A, Botchway F, Addo-Gyan D, Tetteh-Tsifoanya M, Lamptey A, Djameh G, Iqbal SA, Lekpor C, Banerjee S, Wilson MD, Dei-Adomakoh Y, Adjei AA, Stiles JK, Driss A. Int J Mol Sci. 2023 Apr 19;24(8):7546. doi: 10.3390/ijms24087546. PMID: 37108709
Modulation of Heme-Induced Inflammation Using MicroRNA-Loaded Liposomes: Implications for Hemolytic Disorders Such as Malaria and Sickle Cell Disease. Bashi A, Lekpor C, Hood JL, Thompson WE, Stiles JK, Driss A. Int J Mol Sci. 2023 Nov 29;24(23):16934. doi: 10.3390/ijms242316934. PMID: 38069257
https://www.linkedin.com/in/alaijah-bashi-a453b3b3/
Former grad Students

Keri Oxendine Harp, PhD, MSCR: Keri Oxendine Harp, Ph.D., MSCR, currently serving as a Parasitologist at the CDC Foundation, is a dedicated researcher with a strong background in biomedical sciences. With a Ph.D. and MSCR from Morehouse School of Medicine and a Bachelor’s degree in Biology from Mars Hill University, Keri brings a wealth of knowledge and expertise to her role. Her research contributions, particularly in the field of malaria and sickle cell disease, are significant, as evidenced by her numerous publications in reputable journals. Keri’s skills in scientific communication, manuscript writing, and presentations have been instrumental in disseminating her research findings effectively. Her experience in clinical research coordination and laboratory experimentation further demonstrates her proficiency in executing complex research projects. Keri’s commitment to advancing public health through her work at the CDC Foundation underscores her passion for making meaningful contributions to global health initiatives.
Upon graduation and at the end of her training in Dr. Driss’ Lab, she published 8 peer reviewed articles, 3 of them as a first author.
Analysis of clinical presentation, hematological factors, self-reported bed net usage, and malaria burden in sickle cell disease patients. EClinicalMedicine. DOI: 10.1016/j.eclinm.2021.101045.
Elevated neuregulin-1β levels correlate with plasma biomarkers of cerebral injury and high stroke risk in children with sickle cell anemia. Endocrine and Metabolic Science. DOI: 10.1016/j.endmts.2021.100088
Hemoglobin genotypes modulate inflammatory response to Plasmodium infection. Frontiers in Immunology. DOI: 10.3389/fimmu.2020.593546.
MiR-451a and let-7i-5p loaded extracellular vesicles attenuate heme-induced inflammation in hiPSC-derived endothelial cells. Front Immunol. DOI: 10.3389/fimmu.2022.1082414.
MicroRNAs miR-451a and let-7i-5p profiles in circulating exosomes vary among individuals with different sickle hemoglobin genotypes and malaria. J. Clin. Med. DOI: 10.3390/jcm11030500.
Modelling heme-mediated brain injury associated with cerebral malaria in human brain cortical organoids. Scientific reports. DOI: 10.1038/s41598-019-55631-8.
Neuregulin-1/ErbB4 signaling modulate Plasmodium falciparum HRP2-induced damage to brain cortical organoids. IScience. DOI: 10.1016/j.isci.2022.104407.
Sickle cell hemoglobin genotypes affect malaria parasite growth and correlate with exosomal miR-451a and let-7i-5p levels. Int J Mol Sci. DOI: 10.3390/ijms24087546.
https://www.linkedin.com/in/kerioxendineharp/

Join the Driss Lab! – we are actively recruiting for all positions
Graduate Students – Those interested in graduate school can apply to the Morehouse School of Medicine Graduate Education in Biomedical Sciences (https://www.umassmed.edu/gsbs/). We are currently accepting rotation students and full time grad students.
Postdoctoral Fellows
Join our vibrant research community at the Driss Lab! We’re on the lookout for passionate and skilled postdocs eager to delve into the intricacies of heme-induced inflammation in malaria and sickle cell disease. Proficiency in organoid generation, miRNA, exosomes, and expertise in molecular and immunology techniques are highly valued. If you’re ready to contribute to cutting-edge biomedical research and make a difference, apply now to embark on this exciting journey with us! In addition to laboratory activities, attendance at national/international conferences, contributions towards manuscript preparation and fellowship/grant writing is encouraged. A minimum time commitment of two years will be required. If interested, please send a cover letter and CV to Dr. Adel Driss at adriss [at] msm.edu.
